 Professional area | Culture factsheet | Feeding | Nutrients N-P-K-Ca-Mg-S-CL
Professional area | Culture factsheet | Feeding | Nutrients N-P-K-Ca-Mg-S-CL
The feeding of cyclamen is governed first and foremost by climatic factors, as well as by the availability of air and water to the roots.
Each of these factors must have its influence on the choice of balanced feeding.
Definition of the basic concepts in proper rationing
According to COMIFER (French Committee on [Plant] Rationing), there are at least 2 notions that must be distinguished:
The need for a nutrient is the quantity of that element taken up by the plants; this quantity is necessary and sufficient for reaching the set production aim.
Not all varieties of cyclamen have the same capacity for extracting nutrients from their surroundings.
There are varieties known as "demanding", which have a low capacity for extracting the nutrients they need from their surrounding.
With these "demanding" varieties, the cyclamen will show a "response" to application of more manure than that used in traditional culture.
Bulb plants, including cyclamen, are classified as demanding of phosphorus and trace elements.
Nitrogen: main agent of production
After water, nitrogen is the main factor in plant growth, and, of all elements absorbed by the roots, accounts for the greatest absolute quantity.
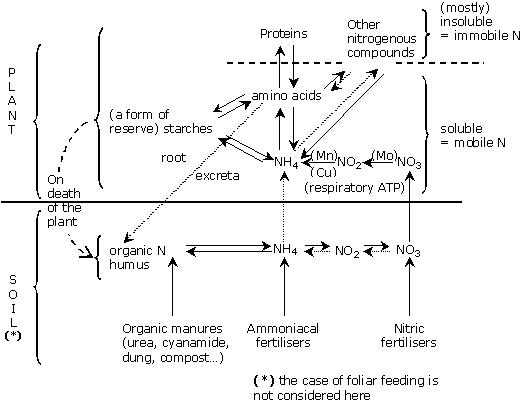
Root nitrogen takeup is by ion absorption.
The 2 ionic forms of nitrogen are:
The relative nutritional affinity of these two main mineral forms – ammoniacal nitrogen and Nitrogen as nitrate – varies depending on:

Note :
All cyclamen can absorb organic nitrogen, in small quantities, in the form of small organic molecules; but this is always on a very small scale compared with other means. It is particularly important when recourse is had to urea foliar dusting.
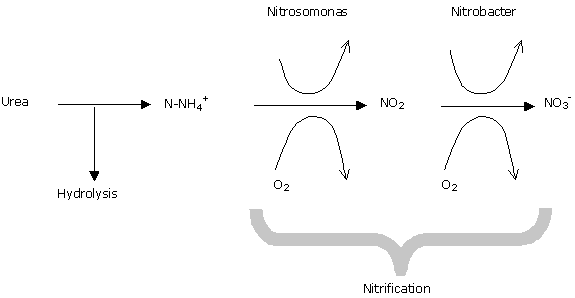
Urea becomes ammoniacal nitrogen by electrolysis, and this is then nitrified into nitric nitrogen, the form assimilable by cyclamen.
The rate at which urea hydrolyses, on the other hand, is quite variable and not easy to control.
Process of decomposition of organic matter: a stage which takes place in environments which are microbiologically active, well watered, and not too acid.
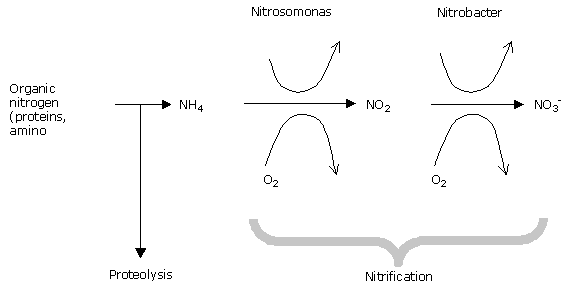
As the diagram shows, the use of organic nitrogen is still governed by many external factors, and this makes timely availability of nitrogen a chancy matter (temperature, oxygen,…) – (This is the case with composts, and organic nitrogenous manures).
Ammoniacal nitrogen is a cation: NH4+. It is therefore fixed in the CEC, in a ratio close to 1 NH4+ fixed in the CEC: 1 NH4+ in aqueous solution. This ratio applies to peaty mediums, and is a slight brake on nitrogen loss by leaching. Some materials (zeolites,…) have a very strong affinity for ammoniacal nitrogen and can store enough of it to give a real "slowing effect".
Watch out, though, for certain releases of ammoniacal nitrogen into the solution (this can happen in active organic environments)…, just when they are not wanted.
A cyclamen which develops in normal conditions of heat and oxygen supply will regulate and control its own nutrition. Nitrogen as nitrate then remains the form predominantly used.
On the other hand, a cyclamen that is stressed, especially at the roots (oxygen and temperature) no longer actively assimilates, only passively. Ammoniacal nitrogen may then be taken up in excessive quantity, and the cyclamen poisons itself with a sap that is too alkaline.
Example:
planting out, and ammoniac poisoning
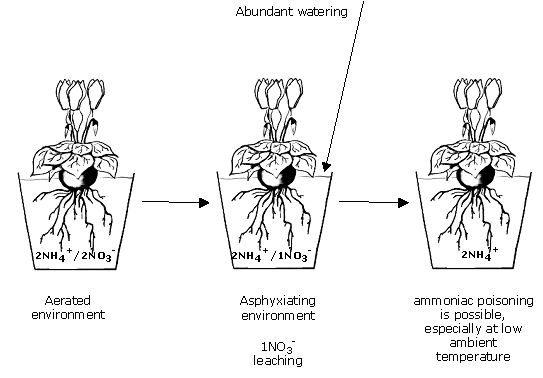
With a peaty medium recently fertilised with PGMIX, the N-NO3/N-NH4 ratio is close to 1:1 in aqueous solution.
A slightly leaching "good watering" at planting out time washes through a proportion of the nitric nitrogen, while the ammoniacal nitrogen, less readily leached, remains in solution.
There is sure to be damage when a second watering is given too soon. Nitrification is blocked, for lack of oxygen in the medium, and the roots’ self-regulation is upset by an over-long inability to breathe.
When we analyse a medium in the course of a growing season, the proportion of ammoniacal nitrogen must be low.
This bears witness to good aeration of the environment, since the nitrifying processes are working properly.
Ammoniacal nitrogen is more liable to be toxic when pH is high.
On the other hand, when pH is low and the environment acidic, the assimilation of calcium is slowed, due to the opposition with ammoniacal nitrogen.
Nitrification is less active under low temperature conditions, but also under low light conditions. It is better, therefore, to lower the ammoniacal proportion in the fertiliser used (and, it follows, the proportion of urea).
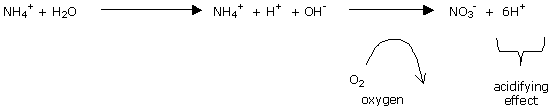
The process of nitrification of ammoniacal nitrogen into nitric nitrogen leads to temporary acidification. This acidification is "soft".
The acidifying effect of ammoniacal nitrogen is not, in this case, used for purposes of a real lowering of pH in organic mediums, but in order to stabilise the pH, or to slow its rise.
The acidifying effect of nitrification is still small, of course, compared with the alkalinisation produced by supplying a strong alkali such as bicarbonates.
In an inert environment, on the other hand, the acidifying effect is a realistic means of control of solution pH.
Nitrogen as nitrate is an anion: NO3-. It is not, therefore, fixed in the CEC (Cation Exchange Capacity).
This component is therefore leachable.
Feeding nitrogen exclusively as nitrate leads to increased medium pH.
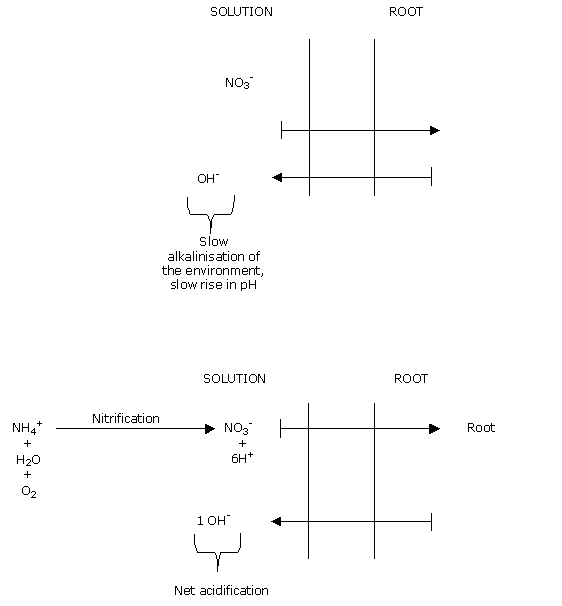
This is why it is always advisable to include a small proportion of ammoniacal nitrogen in all feeding.
Nitrogen is an essential contributor to vigour and vegetative growth.
Nitrates do, of course, encourage the manufacture of dry matter: above all, a gain in total weight.
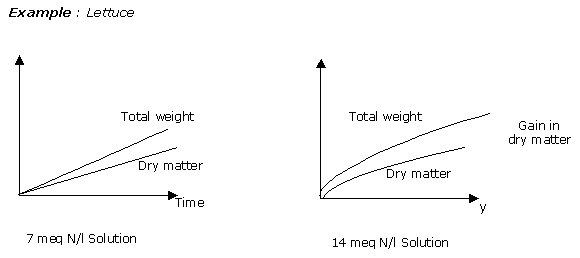
In this example, the supplying of nitrogen and of nitrate does not encourage a dry matter gain but an overall weight gain.
This is the phenomenon known as dilution, well known in cyclamen, with the formation of large leaves.
How is it that making great amounts of nitrate available to the roots encourages a great accumulation of water and contributes to overall weight gain?
The ready availability of water to the cyclamen due to the presence of the nitrates at the roots make the cyclamen soft, frost sensitive, and encourages cell elongation by swelling of the vacuoles.

1st means :
The Nitrogen / Potassium ratio, N / K2O, must shift in favour of potassium. Put simply, potassium is the "master cation", which "chaperones" the nitrogen.
What it in fact does is get consumed in amounts at least equal to, and sometimes even greater than, the nitrogen.
On the other hand, it is taken up in preference to calcium and magnesium.
Moreover, the main form in which potassium is present in the tissues is as an ion: K+ (hydrate).
It can therefore supply the positive charges needed to neutralise the negative charges.
It thus neutralises the organic acids formed by the reduction of the nitrates.
In this way, the operation of the '"water demand” due to the accumulation of nitrates is stopped by the potassium.
Moreover, potassium regulates transpiration, and hence water demand.
Potassium is consequently the cation needed to counterbalance nitrogen and its consequences for elongation and frost sensitiveness.
2nd means :
Controlling vigour by increase in salinity
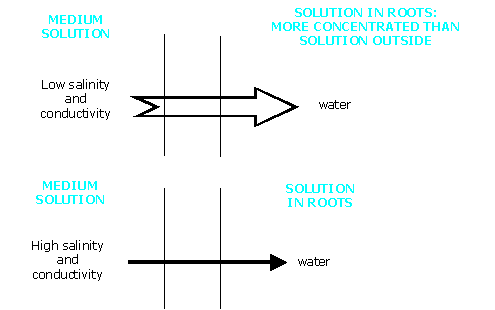
Water always moves from the environment with the lower concentration towards that with the higher. So when the salinity of the medium solution increases, the flow of water through the root becomes less.
It is possible to bring about a real physiological drought for cyclamen by excess salinity.
Now the main agent of salinity in a medium is still Nitrogen.
Control of the water flow by raising salinity with an increase in the concentration of nitrogen is still a delicate technique, but less risky the more suitable the medium.
It is not one recommended for cyclamen, since it leaves the bulb and neck wide open to cryptogam diseases.
Potassium: the master cation
The expression "oxide": K2O does not really apply in an agronomic context, but it is used, for instance in descriptions of fertilisers.
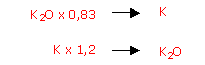
The nutrient, in aqueous solution, is present in the form of electrolytes: K+ and the accompanying anion.
A proportion of the potassium present in the medium is absorbed in the CEC, and this proportion may vary (cf above).
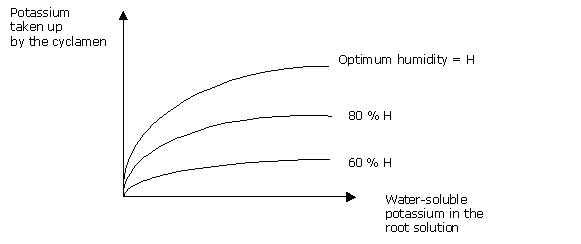
In this way, an essentially “dry” pattern of culture means less takeup of potassium, so that it is necessary to increase the relative percentage of potassium in the nutrition supplied.
In fact the supply of nitrates, phosphates and sulphates through feeding is counterbalanced in the medium by the calcium and magnesium present in the CEC. Potassium, though, is hardly ever displaced in solution by the supply of anions.
It is water mobility which is going to enable a sufficient potassium concentration to be maintained in the root. The K/Ca + Mg ratio is not a satisfactory way of controlling cation supply in an organic medium.
In order, then, to keep an adequate potassium concentration in the medium solution, and ensure good "assimilability" of the potassium, it is necessary to spread out the potassium supplies as much as possible.
Most varieties of cyclamen increase their potassium consumption naturally at the following stages:
At these times, the quantities consumed (or exported) may be three to four times as much as those of nitrogen.
The great readiness of potassium to diffuse through the root allows it to be "over-consumed" on occasion, when its concentration in the environment increases.
Now this over-consumption stimulates cell respiration, and the result, in agronomic terms is:
But of course we must also beware of over-activation of the respiration coefficient , which will fatigue the cyclamen and may lead to early ageing.
What is needed, then, is to keep the N:K2O ratio balanced near 1:2 for the juvenile growth stages, and then let them move up to 1/3 or 1/5 in the pre-maturation phase.
Internal need for potassium increases as the amount of sunshine increases, because the cyclamen is induced to make full use of the greater quantity of radiant energy (this is the case of summer culture of cyclamen).
Potassium requirement is less in winter and at times of little light. The cyclamen consume less potassium than in summer, but we increase the quantity of potassium available to the root so that the small extra uptake of potassium can make the most of the little light there is. In winter, N/K2O rations near 1/3 are recommended.
As a general rule, the more unfavourable the conditions of culture the more the N/K2O ratio should tend toward potassium, especially if medium humidity and rooting are not well controlled.
Localised irrigation leads to low humidity, and rooting is then often localised in the neighbourhood of a sphere of dampness. The water pressure in the medium is less than with a sprinkler system.
Potassium, though, is better taken up if the relative humidity of the medium is high.
It will also be better to feed with a lower N/K2O ratio of drip feeding, than with a sprinkler or underneath watering system.
NB: sprinkler watering systems are not recommended for cyclamen on hygiene grounds.
Phosphorus is present mainly in the form of anions: HPO42- or H2PO4-.The proportions of these two forms in the medium depends on the environment pH.
Expression in terms of phosphoric anhydride (P2O5) corresponds to a calculation method, rather than this being an assimilable form.
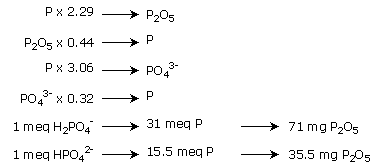
Phosphorus is particularly well taken up within the pH region between 5.5 and 7.0.
Within this pH bracket, phosphorus is mostly present in the medium, fixed by cation bridges.
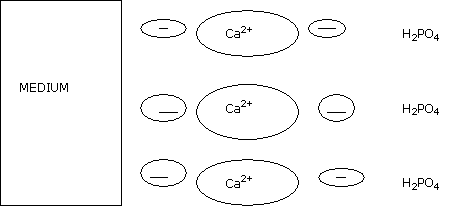
In other words this element is hardly leachable, if at all, on organic mediums.
cf File n°1 : Physiology and nutrition.
The principal synergy concerns nitrogen and phosphorus: N-P. A supply of nitrogen is necessary for better uptake of phosphorus. Typically, this means that a fertiliser such as ammonium phosphate will be useful, not least at times of low temperature. This is because phosphorus uptake is poor at low temperatures, and a combined form of fertiliser, containing both N and P, is used to ensure better use of the phosphorus in adverse conditions.
NB: good phosphorus takeup is necessary for proper movement of reserves towards the bulb.
The principal oppositions in the case of phosphorus concern iron and zinc. In principle, an excess of phosphorus inhibits the takeup of iron and of zinc. Nevertheless, when growing plants in pots in a buffered medium, this phenomenon is hardly ever observed.
Phosphorus encourages molybdenum to enter into solution, in acid environments, and this can contribute, among other things, to a deficiency of molybdenum. This phenomenon is hardly ever observed in pot culture.
Calcium is present in the form of Ca2+ ions in solution. Calcium plays an anti-toxic role, and also one like that of reinforced concrete, for it is essential to the elasticity of the young cells, and slows down cell permeability.
Calcium is mainly influential in all stages of cell growth.
The influence of pH is clearly set out In File n°2: CEC/CALCIUM/BICARBONATES/pH.
Calcium as measured in a water extract is definitely not correlated with calcium assimilable by the plant.
Takeup of calcium depends on four variables:
The more vigorous the variety, the more urgent the immediate needs of the plant. Its affinity for water and nitrogen is higher.
Now calcium is a large cation, and does not move easily through the plant. There may therefore be an imbalance between the immediate calcium need and the need for nitrogen.
There is a higher risk of leaf-edge necrosis.
Calcium is better taken up where waterings are short and frequent. If the cyclamen is showing a “soft” or floppy habit, it is advisable to alter watering in the direction of a smaller quantity, given more often.
The higher the salinity, the less calcium is taken up. For calcium to be transferred properly, all the way to the young leaves and flowers, the flow through the plant that ends in transpiration must be voluminous. High salinity cuts down this transpiratory flow.
The higher the [air] humidity the less well calcium is taken up. High humidity reduces the transpiratory flow through the plant. Calcium then moves only with difficulty and has trouble reaching the young cells.
For calcium to be taken up properly, the medium must be very well aerated, and of fairly low humidity.
The cyclamen shows a soft habit or even leaf-edge necrosis, or flower malformations.
K and Ca are opposed. For regulation of calcium supply to the cyclamen, it may be necessary either to lower the level of potassium in the feeding solution, or to water more frequently, less at a time.
Ca and Mg are only slightly opposed in the case of cyclamen grown in pots. Here the essential thing is to maintain a constant supply of magnesium.
Calcium assimilability is reduced between growing seasons. In these periods the cyclamen’s natural tendency is towards potassium. Moreover, the relative humidity is often higher, and the pattern of watering may not be ideal.
The dangers of leaf-edge necrosis are greater: it is necessary to alter the watering pattern accordingly, by alternating brief waterings with long ones.
Magnesium is present in solution in the form of Mg2+ ions.
Magnesium is an integral part of the chlorophyll molecule. Lack of magnesium therefore shows as a chlorosis of the older leaves.
This is governed by:
Magnesium shows behaviour intermediate between that of calcium and that of potassium, so far as uptake by the roots is concerned.
Most of the absorption of magnesium is correlated with good oxygenation of the medium.
Low temperatures lead to poor uptake of this large atom.
Magnesium is opposed to potassium and to calcium.
In soil-less cultures what is particularly important is to maintain a permanent magnesium concentration at normal levels in the feeding solution.
Most magnesium uptake is governed by water conditions (humidity of the medium / relative humidity of the air / sunshine).
Nitrogen as nitrate N-NO3- and magnesium are synergetic; that is, a supply of nitric nitrogen encourages magnesia uptake.
Sulphur is involved in the synthesis of proteins and of chlorophyll. Sulphur deficiency shows in discoloration of the young parts of the plant, and an accumulation of soluble nitrate in the plant.
There is a close link between sulphur metabolism and that of nitrogen.
Sulphur is present in the form SO4 in aqueous solution. This is the sulphate form.
Certain springs or aquifers have a high sulphate content, sometimes so high as to make this water unusable.
There is a synergy of SO4 with nitrogen. If sulphur availability is inadequate, rising doses of nitrogen lead to lowering of production. This is something observed more and more often on highly fibrous mediums.
Chlorine operates essentially as a brake on transpiration and by restricting the quantity of dry matter produced.
2565, rue de Montourey
83600 Fréjus - France
International telephone : +33 (0)4 94 19 73 04
Switchboard : + 33 (0)4 94 19 73 00
Fax : +33 (0)4 94 19 73 19

