 Professional area | Culture factsheet | Feeding | CEC / pH / Conductivity
Professional area | Culture factsheet | Feeding | CEC / pH / Conductivity
The water given in watering and feeding produces different results depending on the reactivity of the medium; this is described by its CEC (Cation Exchange Capacity) and its power of fixation.
These affect, among other things, the resultant pH and conductivity in the medium.
- Feeding is to be rationed in accordance with the chemical "reactivity" of the medium.
- A medium is described as "reactive" when it alters the medium solution; that is, the solution in which the roots feed.
By their very nature, organic materials and clays have a negative electric charge: they are anions. Cations, on the other hand, are molecules with a positive electric charge.
In the growing medium, the cations are present in two forms:
To simplify, adsorption is very similar to the situation between two magnets :
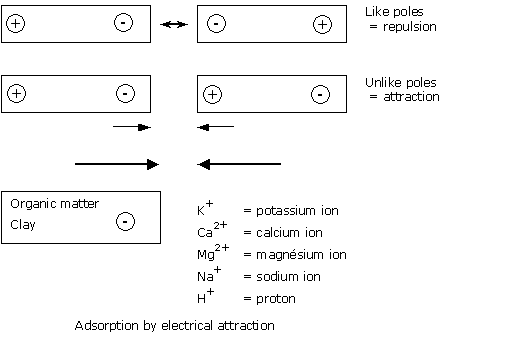
The attractive forces between adsorbed cations and the surface of the organic matter or clay particles are weak. These liaisons can easily be broken.
Because of the weakness of these binding energies, there are constant exchanges between the medium solution and the adsorption sites of the medium.
Mechanisms:
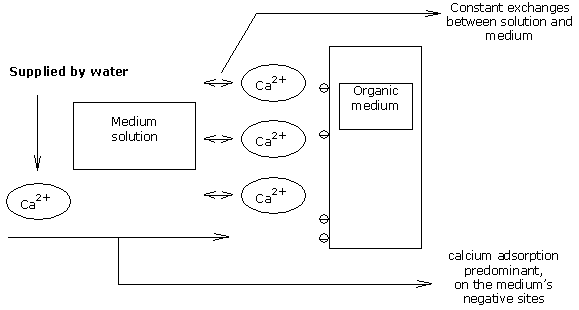
Supplying calcium ions in the medium solution leads to adsorption of calcium onto the medium itself, if there are still fixation sites available.
On a medium of pH 5.5, the CEC. of the medium is not entirely saturated so far as calcium is concerned.
The water’s calcium ions are then adsorbed onto the medium’s available negative sites, up to the point where all such sites are SATURATED.
This is when the saturation point of the CEC is reached.
So the CEC is now saturated in calcium; but does that mean that the pH is stabilised?
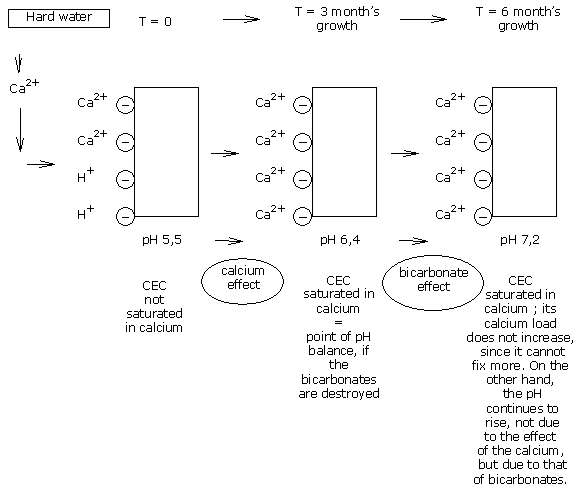
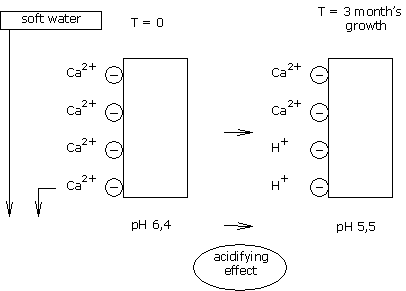
Where hard water is used on a reactive type of medium, calcium is thus part of the CEC’s pH stabilisation.
The bicarbonates are destroyed, but the CEC remains saturated by the calcium ions, which thus allows good stabilisation of the medium pH.
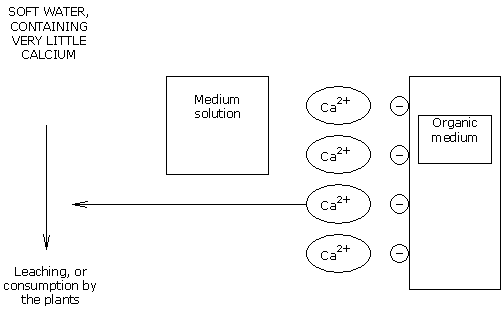
The calcium fixed in the CEC of organic mediums IS RESTORED to the medium solution, in such a way that the same balance is kept between the calcium load in solution and the calcium load in the medium.
The calcium ions lost in the CEC are replaced by H+ ions (protons).
These H+ ions are acidifying, and lower the pH.
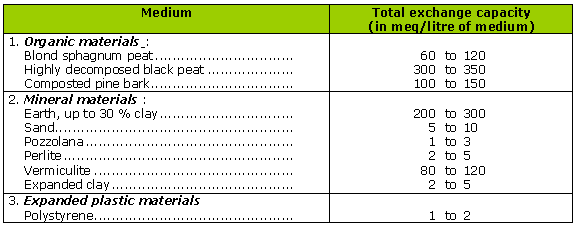
NB: well-developed black peat has at least 3 times the buffering potential of blond peat.

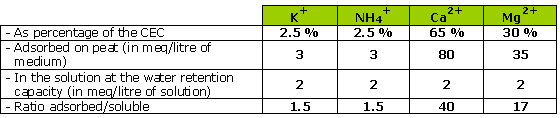
It is to be noted, therefore, that the quantities fixed in the CEC are 40 times higher than those in solution in the case of calcium, and 17 times higher in that of magnesium.
What this means is that calcium and magnesium deficiencies of mediums with pH over 5.5 are not realistic; the same applies to poorly buffered mediums, given the large reserves of these two elements in the CEC.
The "deficiencies", or, rather, poor take-up of calcium and magnesium on conventional organic mediums are "induced": most often by lack of a proper matching of watering and medium.
An example:
Calcium nitrate added to a medium based on blond peat (a poorly buffered medium):
At pH 5.5 (considered acidic), calcium nitrate is supplied for 15 days, 6 times in all, 1 gram/litre.
The saturation rate of the CEC in calcium is near to 50 %
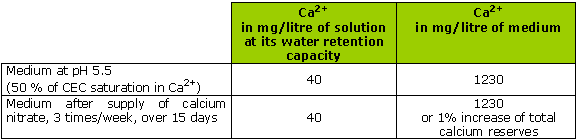
This example shows the illusory aspect of a rapid restoration of pH with calcium nitrate, on any organic medium.
In the case of a medium with an initial pH of 5.5; i.e. 50 % of saturation in calcium and 25 % of saturation in magnesium.
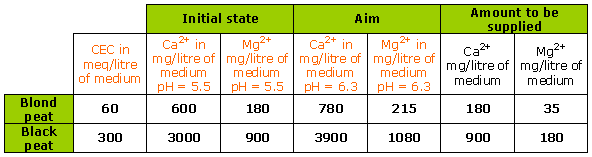
In the case of hard water containing 80 mg/litre Ca2+ (4 meq/litre Ca2+) and 20 mg/litre Mg2+ (1.7 meq/litre Mg2+):
A watering of an average 100 ml/litre of medium provides: 8 mg Ca2+ and 2 mg Mg2+
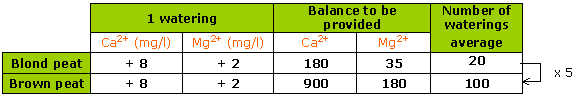
On average, 4 to 5 times more waterings are needed with hard water, for the same effect on pH on a “black” medium as on a “blond” one.
For values of pH lower than 4.5, i.e. for CECs with a saturation rate lower than 30% in Ca2+ and Mg2+, the danger of deficiency is real: this produces blue azaleas, and rules out certain heath plants.
Apart from these extreme cases, and in horticulture in general, it is induced deficiencies that are most often encountered, connected with poor matching of medium and watering.
Note that magnesium especially is more poorly taken up where watering is long and infrequent.
Insufficient root aeration also leads to difficulties in magnesium uptake.
Calcium also is more poorly assimilated where the variety of cyclamen is a vigorous one.
These situations of Ca or Mg deficiency are put right by a change of watering pattern, lowering the humidity in the glasshouse, and reducing the day/night temperature variation.
Potassium is, with ammonium, the cation with the lowest fixation percentage in the CEC; i.e. potassium reserves constitute on average only 2.5 % of the CEC.
Moreover, there is often virtually as much potassium in solution as fixed potassium.
In other words, potassium is an element which is easily leached out.
NB : potassium, in an organic medium, is leached out twice as slowly as nitric nitrogen.
NB also: the ammonium ion has almost the same "retarding” effect as potassium (in the case of which one speaks of reserves!).
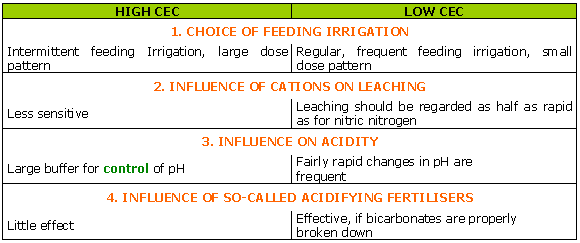
The power of fixation of a medium is its capacity to fix anions and trace elements.
This power of fixation is linked to the presence of "colloid" material, either of organic matter or clay, or to the presence of iron or aluminium hydroxides.
The power of phosphorus fixation of certain soils is known, and is connected with the presence of lime.
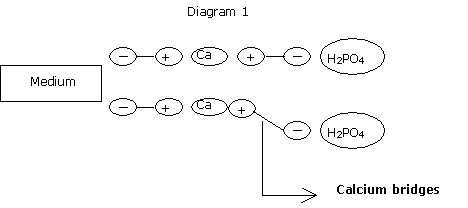
In organic mediums, calcium bridges have little energy.
The power of phosphorus fixation is rather related to the presence of peats rich in aluminium or in iron.
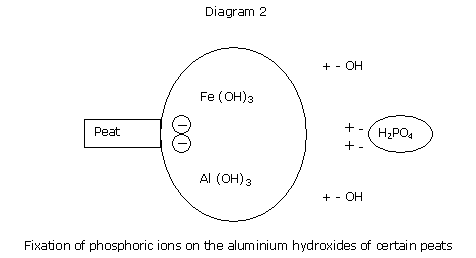
The phosphorus fixation energy for peats can he high, and the phosphates may not be taken up by the cyclamen.
It becomes necessary, then, to saturate all of the aluminium sites with phosphates, so as to ensure that any supply of phosphates in the feeding can still be taken up, and is water soluble.
This is the role of basic, or background feeding.
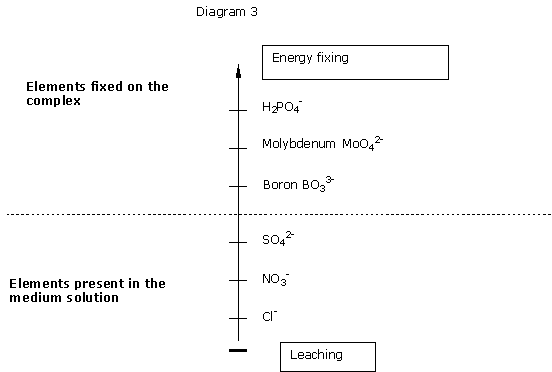
Cl-: chlorides and Nitrogen as nitrate are entirely leachable.
SO42-: the sulphates are leachable also, but have the characteristic that they precipitate in the form of gypsum micro-crystals (CaSO4, 2H2O). These micro-crystals build up, and for a while they lower the sulphate concentration in the surroundings. At the end of a long growing period, this precipitation in gypsum form slows down and the sulphate concentration can rise if the water "driving off" effect is insufficient.
It should be noted, however that sulphate fertilisers increase salinity more than the equivalent nitrates or phosphates, and as a result fertilisers must be chosen which contain little sulphate so as to limit the "immediate" salinity.
BO33-: boron is adsorbed onto organic complexes. It passes rapidly in aqueous solution, and is the more poorly assimilable the more the difference between abundant waterings and drought stress is important. This is often observed in summer.
MoO42-:molybdenum is regarded as not leachable except in an acidic pH.
These trace elements are present in organic mediums essentially in chelated form: the chelates protect the trace elements from being leached out.
Trace elements are not very soluble in water.
Essentially, assimilation takes place by contact between the root and the medium. If this contact is insufficient, assimilation does not take place, and there is a greater risk of deficiency.
It should also be recalled that copper is very strongly fixed by materials liable to fermentation, which can lead to serious deformations.
All biochemical reactions, whether in humans, other animals or plants, are governed by the environment in which they take place.
One of the main factors governing the proper functioning of life processes is the pH of the environment.
The sap of the cyclamen has a pH specific to the species.
The pH is a fundamental feature for mastery of nutrition, because it governs the conditions for take-up of the mineral elements.
pH measures the concentration of hydrogen ions, H+, whence its name of Hydrogen Potential.
It is a measure of the acidity or alkalinity of an environment.
pH = - log [H3O+]

The ratios most often used are: 1/1.5; 1/2.5; the planned European method uses a ratio of 1/5.
pH is highly sensitive to the level of oxygenation of the sample: the more saturated this is in CO2 (carbon dioxide), the higher the value of water pH.
The higher the salinity of the medium, the lower the water pH.
The divergence between water pH and KCl pH is therefore very slight. In fact, the presence of salts, mildly acidic or mildly alkaline fertilisers buffer the behaviour of the hydrogen.
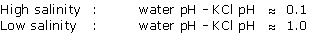
Water pH remains the pH which best reflects the real pH conditions so far as roots are concerned.
This, then, is the measure which the producer will use in the conduct of feeding.
The pH values obtained with this method are always in between the H2O pH and the KCl pH.
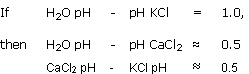
This method, drawn from soil study, consists of making up an extract 1/2.5 with a 1 N solution of KCl.
This pH indicates the degree of saturation of the CEC and is fairly independent of surrounding conditions.
This pH therefore allows us a good view of the evolution of the medium’s saturation or non-saturation in Ca and Mg.
Example :
Measurement of the culture at time T:
15 days later the conductivity has gone down, following suitable waterings:
The KCl pH is still reporting a high degree of saturation of the CEC, and the water pH gives a more coherent value of 6.9, which is moving into the danger area for this culture.
Poor pH control is a regular factor in mishaps in cyclamen.

Aluminium or even manganese poisoning is to be traced to acid pH, in the presence of unstable materials liable to ferment.
Fermentation increases the reduction of manganese and aluminium into forms which then become soluble and potentially poisonous. The phenomenon is then made worse by root asphyxia (lack of oxygen associated with the fermentation).
This poisoning phenomenon frequently arises when the pH goes down during growth and conductivity increases.
Molybdenum deficiency :
Molybdenum deficiency in an acid pH is rarer.
At high pH, the poor availability of phosphorus is little noticed, and tends to show itself in poor plant productivity.
At high pH, as soon as pH nears 7.1 to 7.2, boron deficiency is a reality.
This is often observed in summer. Not only is boron less available at these pH levels, but also the overall water pattern is often defective, and this inhibits the take-up of boron: this often happens with cyclamens.
At high pH, iron and manganese deficiencies are very common in species which are sensitive to iron chlorosis – including, of course, the cyclamen, which as a bulb species has difficulty assimilating these two elements.
Take care! The “hardness” of water, and its consequent effect on the medium, cannot be determined by measuring its pH.
Example : rainwater
Rainwater contains no bicarbonate.
This means it is not buffered.
This water therefore takes the pH of the environment it arose in.
If stored in a reservoir its pH is often near 8.0! .... If fact, its pH is than dependent on the load of water-soluble gases it contains (CO2 in particular).
This rainwater, which leaves its reservoir at pH 8.0, if applied to a medium of pH 5.8 takes this pH value, and leads to a progressive decalcifying of the medium, and thus to acidification.
The "hardness" of water used for watering depends on the content of bicarbonates (HCO3-) and not on the pH.
Bicarbonates are strong alkalis (opposite of strong acids) because they produce a strong rise in du pH.
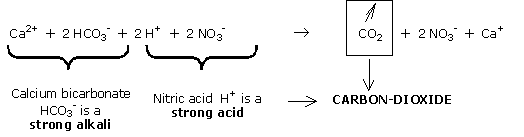
NB : the bicarbonates leave in the form of carbon dioxide; what remains is a balanced weak acid/weak alkali pair: NO3-/Ca2+ in solution.
The pH cannot therefore rise any more.
It is now stable because the calcium always present in the water compensates the losses of Ca2+ in the CEC.
Soft water contains no bicarbonates.
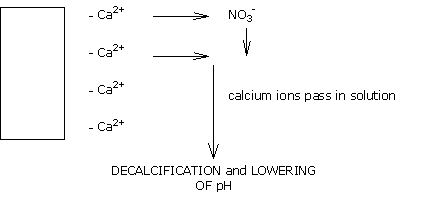
A supplement of CALCIUM NITRATE is then required to compensate for losses of calcium and thus stabilise the pH.
Most of the acidifying features of fertilisers arise from their ammoniacal components.
Fertilisers containing urea, and certain slow-release fertilisers (not indicated for cyclamen),containing urea polymers, are acidifying because the urea turns to ammoniacal nitrogen by hydrolysis.
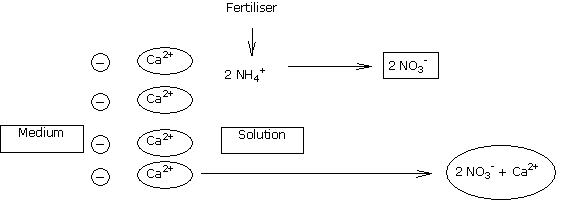
The transformation of ammoniacal nitrogen into nitric nitrogen in aqueous solution leads to passage into solution of a cation Ca2+ (or Mg2+) fixed in the CEC, to balance the solution electrically.
This passage of calcium and magnesium ions into solution encourages the gradual acidification of the environment.
Certain fertilisers supplement the strong acids with the effect of destroying some of the bicarbonates present.
pH and biological stability of the organic materials present
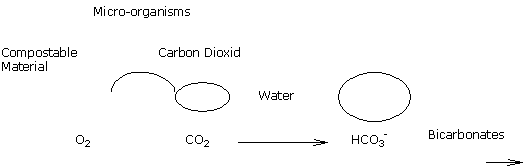
The fermentation of materials such as fresh bark, composts, ... leads to creation of carbon dioxide in the medium’s solution.
A strong partial pressure of carbon dioxide in water encourages the formation of bicarbonates and thus a rise in pH.
Over 6 months’ growing, a mix based on bark which began at a pH of 5.8 can reach pH levels of more than 7.2 by the end of the growth period.
Salinity, expressed in grams per litre, is a measure of the quantity of salts in the medium solution.
It is measured via the electrical conductivity.
The principle of this measurement relies on the property of electrolytes (substances dissociated into ions after dissolving) of conducting electric current more readily, the higher their concentration.
It can be expressed in two ways:
1 - Conductivity, expressed in millisiemens per centimetre (mS/cm)
This is proportional to the salinity.
2 - Resistivity, expressed in ohm-cm
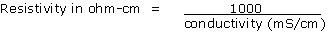
The usual method in France of measuring conductivity is to take an extract of 1/1.5 by volume:
1 volume of medium for 1.5 volumes of demineralised water.
The measurement is taken after 20 minutes of minimum contact, with recommended filtration.
The most immediate effect of the salinity of a medium’s solution is to create an osmotic pressure so great that the cyclamen can no longer absorb water.
This is what is known as physiological drought.
This procedure is used to control vigour.
The vigour of a bulb plant depends above all on the regularity of its factors of production. The more regular the waterings, and the more constant the conductivity, the better will be the plant’s vigour and balance of growth.
With cyclamen in the traditional flowering pot, the conductivity will be quite low.
With mini-cyclamen and in mediums mainly of peat (and thus with a low real coefficient of salinity), conductivity will be high, to keep the plant’s habit compact; for this pattern of watering, with small volumes of medium, gives the plant an ease with respect to water which must be stopped by ensuring higher conductivity.
The measurement of conductivity may be used to discover the nutritive level of the medium.
Example 1 :
Extract: 1/1.5 vol. Measurement of conductivity and nutrients, with a peaty medium enriched with PG Mix at 1 kg/m3


In this example, the measurement of conductivity may be underestimating the nitrogenous proportion.
What happens is that the ammoniacal nitrogen becomes nitric, and the conductivity increases by the same amount, more or less.
Example 2:
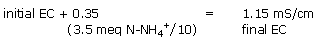

The level of conductivity measured indicates, a priori, a suitable level of nutrition, while the conductivity is only related to a high sulphate content (organic materials manufacture sulphates as they grow older).
With this extract, then, it is better to make a measurement of the nitrogen, with a rapid test, for instance.
Beware of excesses of salts which can lead to susceptibility to disease, degradation of root hairs, and a poor movement of calcium and magnesium, to name but two elements.
2565, rue de Montourey
83600 Fréjus - France
International telephone : +33 (0)4 94 19 73 04
Switchboard : + 33 (0)4 94 19 73 00
Fax : +33 (0)4 94 19 73 19

